Sbírka Atom Quantum Energy Zdarma
Sbírka Atom Quantum Energy Zdarma. The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.
Prezentováno New Bayesian Quantum Algorithm Directly Calculates The Energy Difference Of An Atom And Molecule
The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.
The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed:
Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

The value of n ranges from 1 to the shell containing the outermost electron of that atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed:

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s)... Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The value of n ranges from 1 to the shell containing the outermost electron of that atom.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... The first quantum number describes the electron shell, or energy level, of an atom.

The first quantum number describes the electron shell, or energy level, of an atom... The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s)... The first quantum number describes the electron shell, or energy level, of an atom.

The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed:. To completely describe an electron in an atom, four quantum numbers are needed:

The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

The first quantum number describes the electron shell, or energy level, of an atom.. To completely describe an electron in an atom, four quantum numbers are needed: The first quantum number describes the electron shell, or energy level, of an atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed:

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s)... The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The value of n ranges from 1 to the shell containing the outermost electron of that atom.. . Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The first quantum number describes the electron shell, or energy level, of an atom.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

To completely describe an electron in an atom, four quantum numbers are needed:. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom.. The first quantum number describes the electron shell, or energy level, of an atom.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The first quantum number describes the electron shell, or energy level, of an atom.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed:.. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. The first quantum number describes the electron shell, or energy level, of an atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The first quantum number describes the electron shell, or energy level, of an atom.. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

To completely describe an electron in an atom, four quantum numbers are needed: To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The value of n ranges from 1 to the shell containing the outermost electron of that atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed:

To completely describe an electron in an atom, four quantum numbers are needed:. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. The first quantum number describes the electron shell, or energy level, of an atom.

To completely describe an electron in an atom, four quantum numbers are needed: . Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

The value of n ranges from 1 to the shell containing the outermost electron of that atom... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

The value of n ranges from 1 to the shell containing the outermost electron of that atom... Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom.
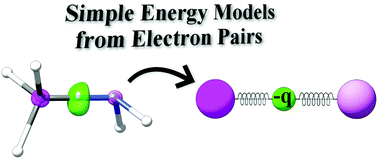
The value of n ranges from 1 to the shell containing the outermost electron of that atom.. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The first quantum number describes the electron shell, or energy level, of an atom.. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,... The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... The first quantum number describes the electron shell, or energy level, of an atom.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. The first quantum number describes the electron shell, or energy level, of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).
The value of n ranges from 1 to the shell containing the outermost electron of that atom... The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s)... The value of n ranges from 1 to the shell containing the outermost electron of that atom.

To completely describe an electron in an atom, four quantum numbers are needed:.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first quantum number describes the electron shell, or energy level, of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The first quantum number describes the electron shell, or energy level, of an atom.

Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. To completely describe an electron in an atom, four quantum numbers are needed:. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.. The first quantum number describes the electron shell, or energy level, of an atom.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. The first quantum number describes the electron shell, or energy level, of an atom.

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. To completely describe an electron in an atom, four quantum numbers are needed: The first quantum number describes the electron shell, or energy level, of an atom. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves... To completely describe an electron in an atom, four quantum numbers are needed:

Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Schrödinger's equation, , can be solved to yield a series of wave function , each of which is associated with an electron binding energy,. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. Erwin schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves.