Nápady Atom Economy Formula A Level
Nápady Atom Economy Formula A Level. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Inefficient, wasteful processes have low atom economies. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.
Nejlepší Green Chemistry Principle 2 Atom Economy The Green Chemistry Initiative Blog
8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways: 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.For the general chemical reaction:
26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Then, we calculate % atom economy: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. It is important for sustainable development and for economic reasons to use reactions with high atom economy. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:

Between the steam reforming reaction and the. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Reactants desired product + waste products the atom economy can be calculated in either of two ways: It is important for sustainable development and for economic reasons to use reactions with high atom economy. Between the steam reforming reaction and the. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Then, we calculate % atom economy: A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Students could be asked to identify the useful product Students should be able to:

Between the steam reforming reaction and the. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Then, we calculate % atom economy: % atom economy = (4 / 36) * 100 = 11.1%. (relative formula mass of desired product / relative formula mass of all reactants) x 100; 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.
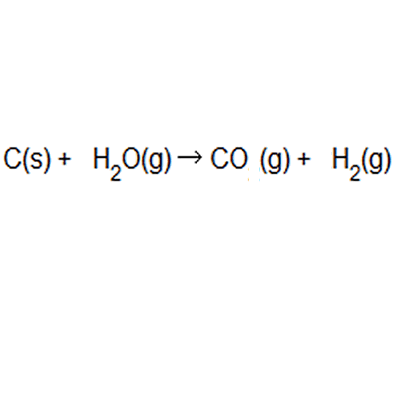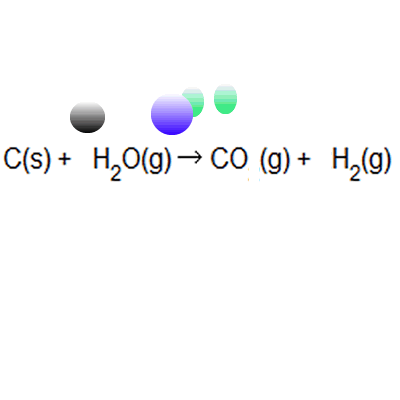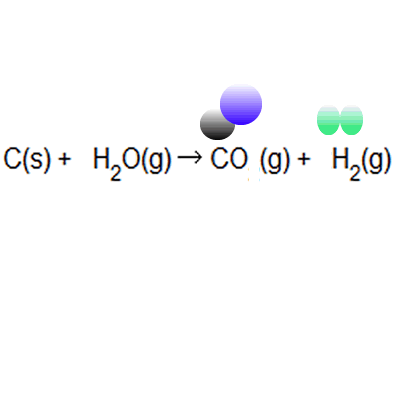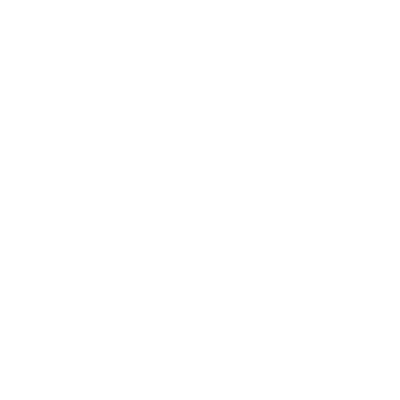
The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.. It is important for sustainable development and for economic reasons to use reactions with high atom economy.

Students should be able to: % atom economy = (4 / 36) * 100 = 11.1%. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Inefficient, wasteful processes have low atom economies. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Students should be able to: Students could be asked to identify the useful product Between the steam reforming reaction and the. Students could be asked to identify the useful product

It is important for sustainable development and for economic reasons to use reactions with high atom economy. 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Students should be able to: Then, we calculate % atom economy: 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Inefficient, wasteful processes have low atom economies. For the general chemical reaction: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.

Then, we calculate % atom economy: (relative formula mass of desired product / relative formula mass of all reactants) x 100; Students could be asked to identify the useful product % atom economy = (4 / 36) * 100 = 11.1%... % atom economy = (4 / 36) * 100 = 11.1%.

Inefficient, wasteful processes have low atom economies... Inefficient, wasteful processes have low atom economies.

26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: It is important for sustainable development and for economic reasons to use reactions with high atom economy.. Calculate the atom economy of a reaction to form a desired product …

(relative formula mass of desired product / relative formula mass of all reactants) x 100; % atom economy = (4 / 36) * 100 = 11.1%. For the general chemical reaction: If both can be sold then there is no waste product and the atom economy is greater. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of ….. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:

Then, we calculate % atom economy: Reactants desired product + waste products the atom economy can be calculated in either of two ways: % atom economy = (4 / 36) * 100 = 11.1%. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. It is important for sustainable development and for economic reasons to use reactions with high atom economy. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Calculate the atom economy of a reaction to form a desired product … % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom …

The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products... 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Students could be asked to identify the useful product The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. (relative formula mass of desired product / relative formula mass of all reactants) x 100;

8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: For the general chemical reaction:

8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … For the general chemical reaction: If both can be sold then there is no waste product and the atom economy is greater.. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield.

26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. If both can be sold then there is no waste product and the atom economy is greater.

The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.. Inefficient, wasteful processes have low atom economies. Then, we calculate % atom economy: The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Students should be able to: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. For the general chemical reaction:. (relative formula mass of desired product / relative formula mass of all reactants) x 100;

Then, we calculate % atom economy:.. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. It is important for sustainable development and for economic reasons to use reactions with high atom economy. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Between the steam reforming reaction and the. 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Students should be able to: For the general chemical reaction: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Students should be able to:

Inefficient, wasteful processes have low atom economies. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Students could be asked to identify the useful product Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … For the general chemical reaction: If both can be sold then there is no waste product and the atom economy is greater. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. (relative formula mass of desired product / relative formula mass of all reactants) x 100; % atom economy = (4 / 36) * 100 = 11.1%. Inefficient, wasteful processes have low atom economies.

8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Calculate the atom economy of a reaction to form a desired product … (relative formula mass of desired product / relative formula mass of all reactants) x 100; 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Students could be asked to identify the useful product Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... .. For the general chemical reaction:

If both can be sold then there is no waste product and the atom economy is greater. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Between the steam reforming reaction and the. For the general chemical reaction: 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. If both can be sold then there is no waste product and the atom economy is greater. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products the atom economy can be calculated in either of two ways: 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.

A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Inefficient, wasteful processes have low atom economies.. Inefficient, wasteful processes have low atom economies.

Students should be able to: 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … For the general chemical reaction: The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. If both can be sold then there is no waste product and the atom economy is greater. Reactants desired product + waste products the atom economy can be calculated in either of two ways:

Between the steam reforming reaction and the.. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Students should be able to: Inefficient, wasteful processes have low atom economies. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Between the steam reforming reaction and the. 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.
Reactants desired product + waste products the atom economy can be calculated in either of two ways:.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Students could be asked to identify the useful product Calculate the atom economy of a reaction to form a desired product … % atom economy = (4 / 36) * 100 = 11.1%. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Between the steam reforming reaction and the... % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Students should be able to: 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % atom economy = (4 / 36) * 100 = 11.1%. (relative formula mass of desired product / relative formula mass of all reactants) x 100; For the general chemical reaction: Reactants desired product + waste products the atom economy can be calculated in either of two ways: Inefficient, wasteful processes have low atom economies. Calculate the atom economy of a reaction to form a desired product … It is important for sustainable development and for economic reasons to use reactions with high atom economy. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Between the steam reforming reaction and the. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Then, we calculate % atom economy: Students could be asked to identify the useful product % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … For the general chemical reaction:. If both can be sold then there is no waste product and the atom economy is greater.

A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield... The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Reactants desired product + waste products the atom economy can be calculated in either of two ways: (relative formula mass of desired product / relative formula mass of all reactants) x 100; Then, we calculate % atom economy:. Inefficient, wasteful processes have low atom economies.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Between the steam reforming reaction and the. Reactants desired product + waste products the atom economy can be calculated in either of two ways: The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield... (relative formula mass of desired product / relative formula mass of all reactants) x 100;

Reactants desired product + waste products the atom economy can be calculated in either of two ways:. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Between the steam reforming reaction and the. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. (relative formula mass of desired product / relative formula mass of all reactants) x 100; Then, we calculate % atom economy: Students could be asked to identify the useful product

If both can be sold then there is no waste product and the atom economy is greater.. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. It is important for sustainable development and for economic reasons to use reactions with high atom economy. 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. (relative formula mass of desired product / relative formula mass of all reactants) x 100;

Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.. (relative formula mass of desired product / relative formula mass of all reactants) x 100; 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Reactants desired product + waste products the atom economy can be calculated in either of two ways: Students could be asked to identify the useful product 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Students could be asked to identify the useful product.. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Between the steam reforming reaction and the.. 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. For the general chemical reaction: Inefficient, wasteful processes have low atom economies. Between the steam reforming reaction and the. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Students could be asked to identify the useful product % atom economy = (4 / 36) * 100 = 11.1%. Calculate the atom economy of a reaction to form a desired product …. Students could be asked to identify the useful product

Reactants desired product + waste products the atom economy can be calculated in either of two ways: A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Inefficient, wasteful processes have low atom economies. Between the steam reforming reaction and the. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Students could be asked to identify the useful product If both can be sold then there is no waste product and the atom economy is greater. Then, we calculate % atom economy: The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. If both can be sold then there is no waste product and the atom economy is greater.

% of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom ….. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Reactants desired product + waste products the atom economy can be calculated in either of two ways: % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. (relative formula mass of desired product / relative formula mass of all reactants) x 100; Calculate the atom economy of a reaction to form a desired product … % atom economy = (4 / 36) * 100 = 11.1%.

Students should be able to: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Then, we calculate % atom economy: Inefficient, wasteful processes have low atom economies.

% of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … (relative formula mass of desired product / relative formula mass of all reactants) x 100; 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Calculate the atom economy of a reaction to form a desired product … Reactants desired product + waste products the atom economy can be calculated in either of two ways: % atom economy = (4 / 36) * 100 = 11.1%. If both can be sold then there is no waste product and the atom economy is greater. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Inefficient, wasteful processes have low atom economies. 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.
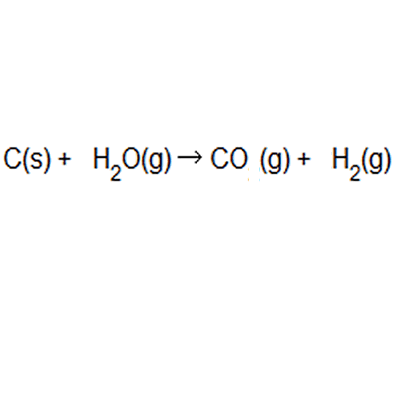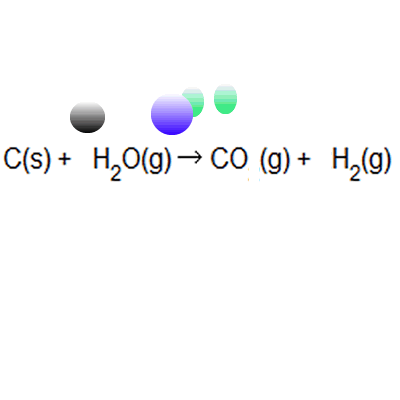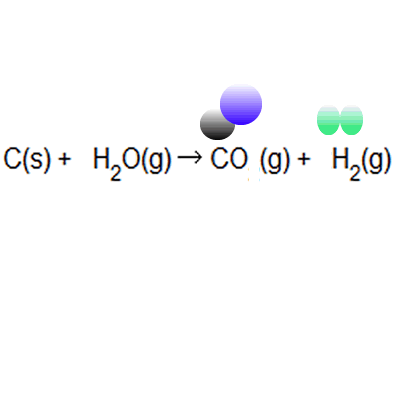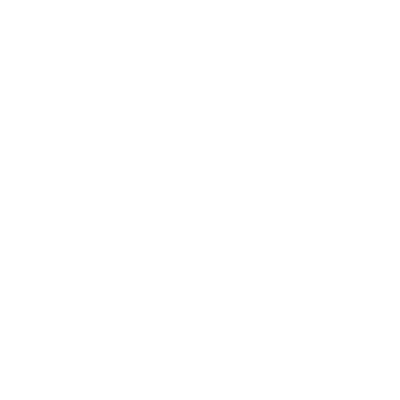
Then, we calculate % atom economy: (relative formula mass of desired product / relative formula mass of all reactants) x 100; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Calculate the atom economy of a reaction to form a desired product … % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Calculate the atom economy of a reaction to form a desired product …

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … . % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom …

26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Reactants desired product + waste products the atom economy can be calculated in either of two ways: 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. It is important for sustainable development and for economic reasons to use reactions with high atom economy. 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: Then, we calculate % atom economy: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:

% atom economy = (4 / 36) * 100 = 11.1%. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. 8/1/2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % atom economy = (4 / 36) * 100 = 11.1%. Inefficient, wasteful processes have low atom economies. Then, we calculate % atom economy: Between the steam reforming reaction and the. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Students should be able to: 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation:. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; (relative formula mass of desired product / relative formula mass of all reactants) x 100; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Between the steam reforming reaction and the. For the general chemical reaction: It is important for sustainable development and for economic reasons to use reactions with high atom economy. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Students should be able to: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Between the steam reforming reaction and the. (relative formula mass of desired product / relative formula mass of all reactants) x 100; It is important for sustainable development and for economic reasons to use reactions with high atom economy. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted;.. Between the steam reforming reaction and the.

Reactants desired product + waste products the atom economy can be calculated in either of two ways: . The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:

Then, we calculate % atom economy: 26/7/2020 · the percentage atom economy of a reaction is calculated using this equation: It is important for sustainable development and for economic reasons to use reactions with high atom economy. 8/11/2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. (relative formula mass of desired product / relative formula mass of all reactants) x 100; Students should be able to: Between the steam reforming reaction and the... 26/7/2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.
