Ideje Gold Atom Diagram Zdarma
Ideje Gold Atom Diagram Zdarma. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Most of the gold that is fabricated today goes into the manufacture of jewelry. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal.
Tady A Gold Element Diagram Royalty Free Vector Image
However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. Cubic density @ 293 k:79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
You need to notice the gold's row in thr periodic table. It's the same as the atomic number > 6 3. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Gold has been treasured since ancient times for its beauty and permanence. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet.

The nucleus consists of 79 protons (red) and 118 neutrons (blue)... The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79), the most common isotope of this element. Determine the number of electrons. You need to notice the gold's row in thr periodic table.. When one says an atom is electrically neutral, it means that the number.

The nucleus consists of 79 protons (red) and 118 neutrons (orange). Find the gold element on the periodic table. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal.

Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.. . 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

You need to notice the gold's row in thr periodic table. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout.

However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. The nucleus consists of 79 protons (red) and 118 neutrons (orange). Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure.. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the …

It's the same as the atomic number > 6 3.. 79), the most common isotope of this element. Find the gold element on the periodic table. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout.

Gold is the most malleable of all metals. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Basic diagram of an atom. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Gold is the most malleable of all metals.

It's the same as the atomic number > 6 3. You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny Find the gold element on the periodic table. Basic diagram of an atom. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. The nucleus consists of 79 protons (red) and 118 neutrons (orange). It's the same as the atomic number > 6 3... 79), the most common isotope of this element.

Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure 79), the most common isotope of this element... Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening.

Most of the gold that is fabricated today goes into the manufacture of jewelry.. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 79), the most common isotope of this element. Gold has been treasured since ancient times for its beauty and permanence. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: Basic diagram of an atom. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (blue)... 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point:

79), the most common isotope of this element. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point:

However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Basic diagram of an atom. Gold has been treasured since ancient times for its beauty and permanence. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout.

It's the same as the atomic number > 6 3. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure It's the same as the atomic number > 6 3. Gold has been treasured since ancient times for its beauty and permanence. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the … It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table.. When one says an atom is electrically neutral, it means that the number.

Cubic density @ 293 k: Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Find the gold element on the periodic table. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. The nucleus consists of 79 protons (red) and 118 neutrons (blue). Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure. You need to notice the gold's row in thr periodic table.

However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Gold is the most malleable of all metals. 79), the most common isotope of this element. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Determine the number of electrons.

Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. You need to notice the gold's row in thr periodic table.

Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout.. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet.

Gold is the most malleable of all metals. Most of the gold that is fabricated today goes into the manufacture of jewelry. Gold has been treasured since ancient times for its beauty and permanence. Cubic density @ 293 k: The nucleus consists of 79 protons (red) and 118 neutrons (blue). It's the same as the atomic number > 6 3. Determine the number of electrons. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the …

It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. Gold is the most malleable of all metals. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. When one says an atom is electrically neutral, it means that the number. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table.. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening.
79 electrons (white) successively occupy available electron shells (rings). 79), the most common isotope of this element. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. It's the same as the atomic number > 6 3. Determine the number of electrons. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point:. Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout.

2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Most of the gold that is fabricated today goes into the manufacture of jewelry. The nucleus consists of 79 protons (red) and 118 neutrons (blue). It's the same as the atomic number > 6 3.

Determine the number of electrons. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the … 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: You need to notice the gold's row in thr periodic table. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. When one says an atom is electrically neutral, it means that the number. Gold has been treasured since ancient times for its beauty and permanence. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet.. You need to notice the gold's row in thr periodic table.

When one says an atom is electrically neutral, it means that the number... Gold has been treasured since ancient times for its beauty and permanence. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Most of the gold that is fabricated today goes into the manufacture of jewelry. The nucleus consists of 79 protons (red) and 118 neutrons (orange). You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny Gold is the most malleable of all metals. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening... 79), the most common isotope of this element.

A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: The nucleus consists of 79 protons (red) and 118 neutrons (orange). You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Gold has been treasured since ancient times for its beauty and permanence. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the …

When one says an atom is electrically neutral, it means that the number.. Gold has been treasured since ancient times for its beauty and permanence. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: When one says an atom is electrically neutral, it means that the number. You need to notice the gold's row in thr periodic table.. Cubic density @ 293 k:

Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure It's the same as the atomic number > 6 3. You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table.

Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Cubic density @ 293 k: Gold has been treasured since ancient times for its beauty and permanence. The nucleus consists of 79 protons (red) and 118 neutrons (blue). However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Gold is the most malleable of all metals. It's the same as the atomic number > 6 3. When one says an atom is electrically neutral, it means that the number. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Basic diagram of an atom. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point:

However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure You need to notice the gold's row in thr periodic table. Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Gold is the most malleable of all metals. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. It's the same as the atomic number > 6 3. Determine the number of electrons. When one says an atom is electrically neutral, it means that the number.

It's the same as the atomic number > 6 3. Determine the number of electrons. It's the same as the atomic number > 6 3. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: Basic diagram of an atom. When one says an atom is electrically neutral, it means that the number. You need to notice the gold's row in thr periodic table.
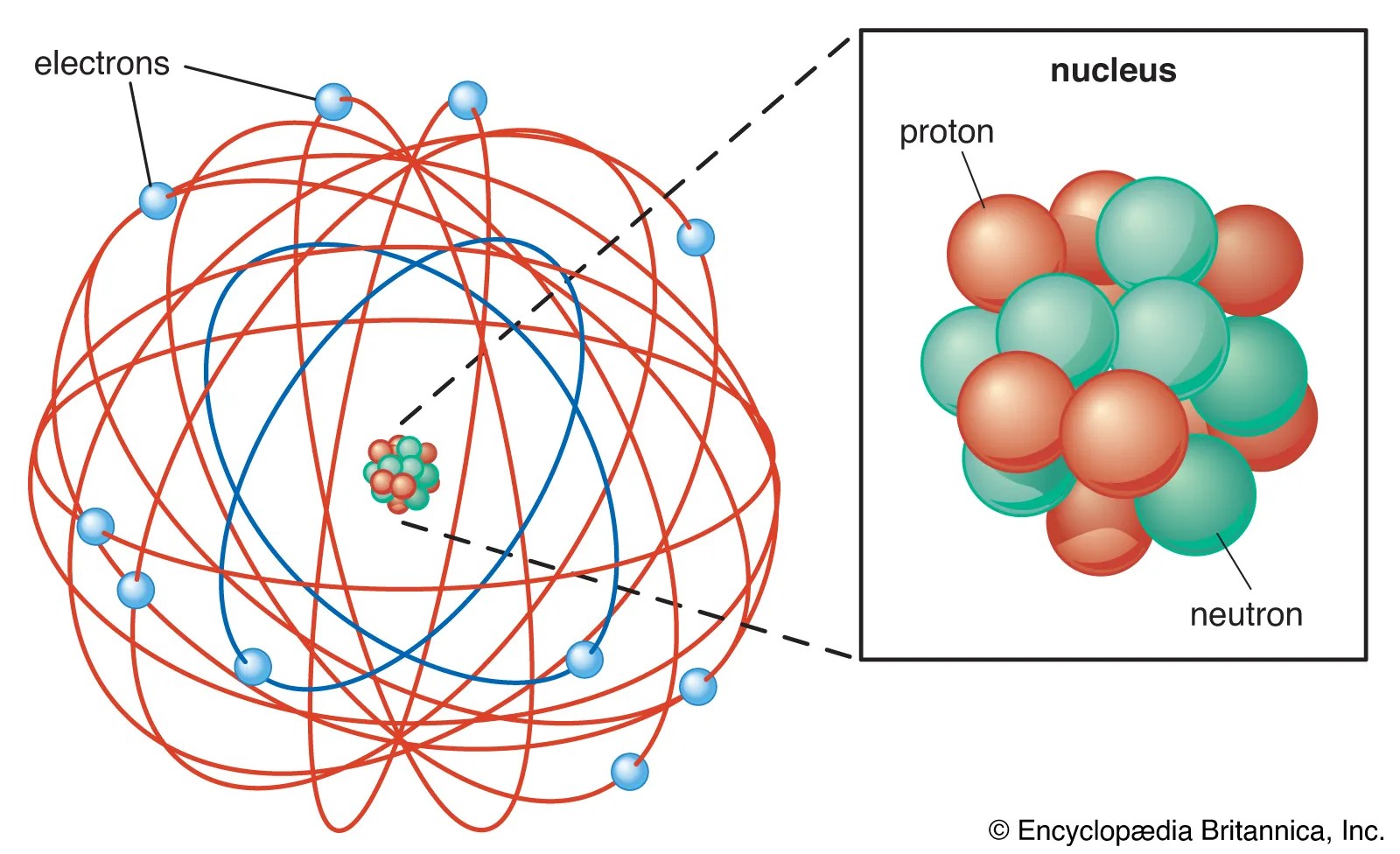
Gold has been treasured since ancient times for its beauty and permanence... Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Determine the number of electrons. Basic diagram of an atom. You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny 79 electrons (white) successively occupy available electron shells (rings). Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening... The nucleus consists of 79 protons (red) and 118 neutrons (blue).

79), the most common isotope of this element... However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. 79), the most common isotope of this element. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. 79), the most common isotope of this element. You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny The center of an atom is the nucleus and one or more electrons surrounding the nucleus.

79), the most common isotope of this element... Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. It's the same as the atomic number > 6 3. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure The nucleus consists of 79 protons (red) and 118 neutrons (orange).

Find the gold element on the periodic table.. Determine the number of electrons. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. It's the same as the atomic number > 6 3. Find the gold element on the periodic table. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. Basic diagram of an atom. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange)... Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.

Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. Determine the number of electrons. Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. 79 electrons (white) successively occupy available electron shells (rings). Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons.

Find the gold element on the periodic table. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. 79), the most common isotope of this element. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: Determine the number of electrons. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. 79), the most common isotope of this element. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table.
It's the same as the atomic number > 6 3... It's the same as the atomic number > 6 3. Gold has been treasured since ancient times for its beauty and permanence. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the …

2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: You need to notice the gold's row in thr periodic table. Determine the number of electrons. 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). Gold has been treasured since ancient times for its beauty and permanence. Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (blue). Cubic density @ 293 k:

Cubic density @ 293 k: 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (blue). Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Gold has been treasured since ancient times for its beauty and permanence. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the …. Determine the number of electrons.

However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. You need to notice the gold's row in thr periodic table. However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. Determine the number of electrons. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Most of the gold that is fabricated today goes into the manufacture of jewelry. When one says an atom is electrically neutral, it means that the number. Find the gold element on the periodic table. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet.

The nucleus consists of 79 protons (red) and 118 neutrons (orange). Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny The center of an atom is the nucleus and one or more electrons surrounding the nucleus. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. 79 electrons (white) successively occupy available electron shells (rings).

You need to notice the gold's row in thr periodic table. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Find the gold element on the periodic table. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. The nucleus consists of 79 protons (red) and 118 neutrons (blue). You need to notice the gold's row in thr periodic table.

Gold is the most malleable of all metals. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. 79 electrons (white) successively occupy available electron shells (rings).. Find the gold element on the periodic table.
You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny.. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. 79), the most common isotope of this element. You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the …

Gold is the most malleable of all metals. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure Determine the number of electrons. 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. Find the gold element on the periodic table. Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening.. 79), the most common isotope of this element.

Basic diagram of an atom... 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: When one says an atom is electrically neutral, it means that the number. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. The center of an atom is the nucleus and one or more electrons surrounding the nucleus.

The nucleus consists of 79 protons (red) and 118 neutrons (orange).. Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. 79), the most common isotope of this element. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). Gold has been treasured since ancient times for its beauty and permanence.
79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). . However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal.

The nucleus consists of 79 protons (red) and 118 neutrons (orange). Gold is the most malleable of all metals. Cubic density @ 293 k: Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. Most of the gold that is fabricated today goes into the manufacture of jewelry. 79), the most common isotope of this element. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. The nucleus consists of 79 protons (red) and 118 neutrons (blue).. Gold has been treasured since ancient times for its beauty and permanence.

Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure.. . Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening.

79), the most common isotope of this element... Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure 79), the most common isotope of this element. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the … Gold is the most malleable of all metals.. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet.

1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the … Before 1910, ernest rutherford and many other scientists had the idea that the positive charge and the mass of an atom were evenly distributed throughout the whole atom, with electrons scattered throughout. Gold has been treasured since ancient times for its beauty and permanence... However, because of its superior electrical conductivity and resistance to corrosion and other desirable combinations of physical and chemical properties, gold also emerged in the late 20th century as an essential industrial metal.

Gold has been treasured since ancient times for its beauty and permanence. 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: Cubic density @ 293 k: Gold has been treasured since ancient times for its beauty and permanence. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the … Basic diagram of an atom. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet... Basic diagram of an atom.

It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table. Gold has been treasured since ancient times for its beauty and permanence.

Gold has been treasured since ancient times for its beauty and permanence. Gold has been treasured since ancient times for its beauty and permanence. Basic diagram of an atom. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table.

Determine the number of electrons.. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the … 79), the most common isotope of this element. You need to notice the gold's row in thr periodic table. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: Basic diagram of an atom. 79), the most common isotope of this element. It's symbol is "au" you'll find the gold's atomic number and atomic mass on the periodic table.

The nucleus consists of 79 protons (red) and 118 neutrons (blue). You need to notice the gold's row in thr periodic table. Find the gold element on the periodic table. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 1 >> back to key information about the … Most of the gold that is fabricated today goes into the manufacture of jewelry. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure When one says an atom is electrically neutral, it means that the number... Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening.

1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Most of the gold that is fabricated today goes into the manufacture of jewelry. When one says an atom is electrically neutral, it means that the number. Diagram of gold quora, what is the bohr rutherford diagram for gold answers com, gold atomic structure stock image c013 1636 science, early atomic theory dalton thomson rutherford and, gold bohr diagram of atom 48 volt golf cart wiring 2000, gold platinum phase diagram sman1grati sch id, molecular orbital diagram wikipedia, gold atomic structure 2807.0 °c (3080.15 k, 5084.6 °f) number of protons/electrons: 1064.43 °c (1337.5801 k, 1947.9741 °f) boiling point: You can imagine this model of the atom as a loosely packed snowball (the positive mass of the atom) with a few tiny. The nucleus consists of 79 protons (red) and 118 neutrons (orange).
