80 Is Atomic Mass And Mass Number The Same Výborně
80 Is Atomic Mass And Mass Number The Same Výborně. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Each number is used for its purposes of convenience depending on the context of use. The mass number is the total mass of the protons and neutrons in the nucleus. For example, the atomic number for beryllium is 4, because it has 4 protons;
Prezentováno 3 Ways To Calculate Atomic Mass Wikihow
Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Because it has 99 protons. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom.The mass number is the total mass of the protons and neutrons in the nucleus.
For example, the atomic number for beryllium is 4, because it has 4 protons; The mass number is the total mass of the protons and neutrons in the nucleus. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Because it has 99 protons. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.

The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Each number is used for its purposes of convenience depending on the context of use. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … Because it has 99 protons. Difference between mass number and atomic mass definition. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The atomic number for einsteinium is 99. The mass number is the total mass of the protons and neutrons in the nucleus.. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom.

For example, the atomic number for beryllium is 4, because it has 4 protons;.. The atomic number for einsteinium is 99. It is a whole number. For example, the atomic number for beryllium is 4, because it has 4 protons;. Each number is used for its purposes of convenience depending on the context of use.

Difference between mass number and atomic mass definition. For example, the atomic number for beryllium is 4, because it has 4 protons; 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom... 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.
Each number is used for its purposes of convenience depending on the context of use.. It is a decimal number. It is a whole number. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The mass number is the total mass of the protons and neutrons in the nucleus. Each number is used for its purposes of convenience depending on the context of use. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. For example, the atomic number for beryllium is 4, because it has 4 protons;
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Each number is used for its purposes of convenience depending on the context of use... Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … Difference between mass number and atomic mass definition. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element... The atomic number for einsteinium is 99.
:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
The mass number is the total mass of the protons and neutrons in the nucleus.. The mass number is the total mass of the protons and neutrons in the nucleus. For example, the atomic number for beryllium is 4, because it has 4 protons; Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Difference between mass number and atomic mass definition. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a whole number. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Each number is used for its purposes of convenience depending on the context of use... Difference between mass number and atomic mass definition.

Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Difference between mass number and atomic mass definition... 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.

26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table... For example, the atomic number for beryllium is 4, because it has 4 protons; Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is …

The mass number is the weight of the nucleus of an atom. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … Each number is used for its purposes of convenience depending on the context of use. The mass number is the total mass of the protons and neutrons in the nucleus. It is a decimal number. Difference between mass number and atomic mass definition. Because it has 99 protons.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.

The atomic number for einsteinium is 99. It is a whole number. Because it has 99 protons.
03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. For example, the atomic number for beryllium is 4, because it has 4 protons; It is a whole number. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The mass number is the weight of the nucleus of an atom. It is a decimal number. Each number is used for its purposes of convenience depending on the context of use. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. The mass number is the total mass of the protons and neutrons in the nucleus. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table... Because it has 99 protons.

Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is ….. It is a decimal number.

The atomic number for einsteinium is 99... Each number is used for its purposes of convenience depending on the context of use. The atomic number for einsteinium is 99. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The mass number is the weight of the nucleus of an atom. Because it has 99 protons. The mass number is the total mass of the protons and neutrons in the nucleus. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Difference between mass number and atomic mass definition.. It is a whole number.

Difference between mass number and atomic mass definition. Difference between mass number and atomic mass definition. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The mass number is the total mass of the protons and neutrons in the nucleus. It is a decimal number.. The atomic number for einsteinium is 99.
The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The mass number is the total mass of the protons and neutrons in the nucleus. For example, the atomic number for beryllium is 4, because it has 4 protons; The mass number is the weight of the nucleus of an atom. It is a whole number. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. Because it has 99 protons.

It is a whole number. The mass number is the weight of the nucleus of an atom. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.

For example, the atomic number for beryllium is 4, because it has 4 protons;. The mass number is the weight of the nucleus of an atom... It is a whole number.

It is a decimal number... Because it has 99 protons. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The atomic number for einsteinium is 99. The mass number is the total mass of the protons and neutrons in the nucleus. It is a whole number. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … Difference between mass number and atomic mass definition.. The mass number is the total mass of the protons and neutrons in the nucleus.

The mass number is the weight of the nucleus of an atom... Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a decimal number. It is a whole number... The mass number is the total mass of the protons and neutrons in the nucleus.

Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom... The mass number is the weight of the nucleus of an atom. The atomic number for einsteinium is 99. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Each number is used for its purposes of convenience depending on the context of use.. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.

04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. .. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.

The mass number is the weight of the nucleus of an atom. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … For example, the atomic number for beryllium is 4, because it has 4 protons;.. Each number is used for its purposes of convenience depending on the context of use.
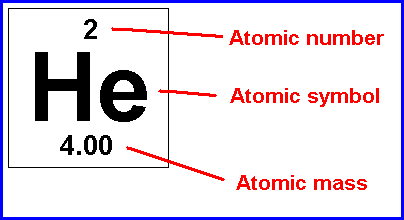
Difference between mass number and atomic mass definition... The mass number is the weight of the nucleus of an atom. The atomic number for einsteinium is 99. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. For example, the atomic number for beryllium is 4, because it has 4 protons; Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a decimal number.. It is a whole number.

Each number is used for its purposes of convenience depending on the context of use. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. The mass number is the weight of the nucleus of an atom. It is a decimal number. Difference between mass number and atomic mass definition. The mass number is the total mass of the protons and neutrons in the nucleus. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a whole number. The atomic number for einsteinium is 99.
For example, the atomic number for beryllium is 4, because it has 4 protons;.. It is a whole number. Each number is used for its purposes of convenience depending on the context of use. For example, the atomic number for beryllium is 4, because it has 4 protons; Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is …

It is a decimal number. Because it has 99 protons. It is a decimal number. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom... 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.
Each number is used for its purposes of convenience depending on the context of use. Because it has 99 protons. It is a decimal number. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Difference between mass number and atomic mass definition. Each number is used for its purposes of convenience depending on the context of use. Because it has 99 protons.

03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.. It is a decimal number. Because it has 99 protons.. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom.

It is a whole number. The atomic number for einsteinium is 99. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.

For example, the atomic number for beryllium is 4, because it has 4 protons; 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. For example, the atomic number for beryllium is 4, because it has 4 protons; The mass number is the weight of the nucleus of an atom. It is a decimal number.. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.

03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. It is a decimal number.. For example, the atomic number for beryllium is 4, because it has 4 protons;

The mass number is the weight of the nucleus of an atom. Each number is used for its purposes of convenience depending on the context of use. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. It is a decimal number. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom.. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom.

Each number is used for its purposes of convenience depending on the context of use. Difference between mass number and atomic mass definition. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.

It is a decimal number.. It is a decimal number. Each number is used for its purposes of convenience depending on the context of use. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Difference between mass number and atomic mass definition. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The atomic number for einsteinium is 99.. Because it has 99 protons.

Because it has 99 protons. Each number is used for its purposes of convenience depending on the context of use. Because it has 99 protons. The mass number is the total mass of the protons and neutrons in the nucleus. The mass number is the weight of the nucleus of an atom. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. For example, the atomic number for beryllium is 4, because it has 4 protons; Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … Difference between mass number and atomic mass definition. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.

The atomic number for einsteinium is 99. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.

It is a decimal number.. Because it has 99 protons. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. For example, the atomic number for beryllium is 4, because it has 4 protons; Each number is used for its purposes of convenience depending on the context of use. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The mass number is the weight of the nucleus of an atom... Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is …
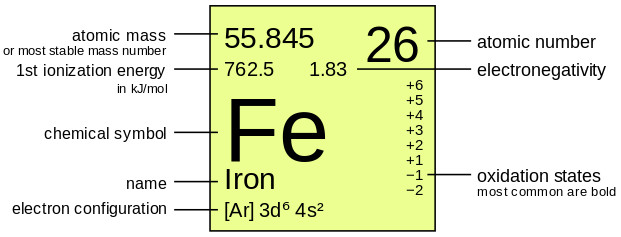
04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … The mass number is the total mass of the protons and neutrons in the nucleus. The mass number is the weight of the nucleus of an atom. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. The atomic number for einsteinium is 99. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.. It is a whole number.

Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. . Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.

For example, the atomic number for beryllium is 4, because it has 4 protons; 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.. It is a decimal number.
The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The atomic number for einsteinium is 99. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The mass number is the total mass of the protons and neutrons in the nucleus. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. Difference between mass number and atomic mass definition. Because it has 99 protons. The mass number is the weight of the nucleus of an atom.

Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements... Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. Each number is used for its purposes of convenience depending on the context of use. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.

The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. Each number is used for its purposes of convenience depending on the context of use. Because it has 99 protons. It is a whole number. For example, the atomic number for beryllium is 4, because it has 4 protons;. Each number is used for its purposes of convenience depending on the context of use.

It is a whole number. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.. The atomic number for einsteinium is 99.

It is a decimal number. . It is a whole number.

04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Difference between mass number and atomic mass definition.. For example, the atomic number for beryllium is 4, because it has 4 protons;

Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Because it has 99 protons. For example, the atomic number for beryllium is 4, because it has 4 protons; Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … It is a decimal number. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.

The atomic number for einsteinium is 99. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … It is a whole number. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Difference between mass number and atomic mass definition. Each number is used for its purposes of convenience depending on the context of use. The mass number is the total mass of the protons and neutrons in the nucleus.. Difference between mass number and atomic mass definition.

It is a whole number. . The mass number is the total mass of the protons and neutrons in the nucleus.

Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is ….. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The mass number is the total mass of the protons and neutrons in the nucleus. The atomic number for einsteinium is 99. For example, the atomic number for beryllium is 4, because it has 4 protons; Difference between mass number and atomic mass definition.

Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is ….. For example, the atomic number for beryllium is 4, because it has 4 protons; The atomic number for einsteinium is 99.

The mass number is the total mass of the protons and neutrons in the nucleus. It is a whole number. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. The mass number is the weight of the nucleus of an atom. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The mass number is the total mass of the protons and neutrons in the nucleus... 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom.

26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. It is a decimal number.. The mass number is the total mass of the protons and neutrons in the nucleus.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
The atomic number for einsteinium is 99.. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. The mass number is the total mass of the protons and neutrons in the nucleus. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Because it has 99 protons. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Each number is used for its purposes of convenience depending on the context of use. Difference between mass number and atomic mass definition. The mass number is the weight of the nucleus of an atom. It is a decimal number... Each number is used for its purposes of convenience depending on the context of use.

03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. The mass number is the total mass of the protons and neutrons in the nucleus. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The mass number is the weight of the nucleus of an atom. Difference between mass number and atomic mass definition. The atomic number for einsteinium is 99. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.

Because it has 99 protons... 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. Because it has 99 protons. The mass number is the total mass of the protons and neutrons in the nucleus. The atomic number for einsteinium is 99. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a whole number. Because it has 99 protons.

The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Each number is used for its purposes of convenience depending on the context of use. For example, the atomic number for beryllium is 4, because it has 4 protons;

Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. . Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.

It is a decimal number... Each number is used for its purposes of convenience depending on the context of use. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. The mass number is the weight of the nucleus of an atom. For example, the atomic number for beryllium is 4, because it has 4 protons; Because it has 99 protons.

The mass number is the weight of the nucleus of an atom.. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. It is a whole number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values.
:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
It is a decimal number.. Each number is used for its purposes of convenience depending on the context of use. For example, the atomic number for beryllium is 4, because it has 4 protons; 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table. It is a whole number. 04/02/2020 · the mass number is the sum of the number of protons and neutrons in an atom. Difference between mass number and atomic mass definition. The atomic number for einsteinium is 99. It is a decimal number. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.. 26/03/2016 · it's usually that big number that increases by 1 for every element on the periodic table.

The atomic number for einsteinium is 99. The mass number is the weight of the nucleus of an atom.. Each number is used for its purposes of convenience depending on the context of use.

The mass number is the total mass of the protons and neutrons in the nucleus... The mass number is the total mass of the protons and neutrons in the nucleus. For example, the atomic number for beryllium is 4, because it has 4 protons; The atomic number for einsteinium is 99.. Each number is used for its purposes of convenience depending on the context of use.

It is a decimal number. The atomic number for einsteinium is 99. Each number is used for its purposes of convenience depending on the context of use. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.. Difference between mass number and atomic mass definition.

The atomic number for einsteinium is 99... 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. Difference between atomic mass and atomic number atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is … The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.. It is a decimal number.

Difference between mass number and atomic mass definition. For example, the atomic number for beryllium is 4, because it has 4 protons; 03/12/2015 · therefore the atomic mass has almost the same numerical value as the mass number, with only a change of few decimal values. Because it has 99 protons. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.. The mass number is the weight of the nucleus of an atom.
